Oligonucleotides, or oligos, are at the core of some of the most life-changing molecular biology tools used today. From qPCR to antisense gene therapy, oligos have changed what scientists can achieve through synthetic and molecular biology. As they continue to grow in popularity, new approaches to synthesizing oligos are being devised to make them more accessible and unlock their potential.
While synthesis methods continue to advance, one significant challenge remains: oligo purification. Although application-dependent, oligos generally require high purity to ensure the reproducibility of experiments and enable researchers to better understand reaction pathways. However, complicated syntheses result in significant impurities that must be removed before application.
To accelerate productivity and maximize the profitability of oligo research and development, it is crucial that workflows, including purification, are carefully optimized. But with so many potential clean-up possibilities, it can be difficult to know where to begin. Here, we explore the most common approaches to oligo purification, and explain how the right high performance liquid chromatography (HPLC) system can overcome any current and future challenges you may face and help you achieve more accurate results.
Understanding Oligo Synthesis and Impurities
The most common method to assemble oligos is solid-phase chemical synthesis, which generally requires deprotection, coupling, oxidation, and capping steps. Here, oligos are extended by one nucleic acid per synthesis cycle. In each cycle, failure sequences or shorter chains (“shortmers”) may be formed by bases failing to achieve the nucleotide coupling reaction. Similarly, low-molecular-weight impurities are produced as a by-product of cleavage and deprotection reactions. For modified oligo synthesis (e.g., labeled or dual-labeled oligos), unmodified oligos with the same sequence length may remain in the crude mixture alongside other residual reagents, such as free dyes, causing significant complications.
To avoid disruption in the subsequent applications of oligos, it is essential to eliminate impurities. The complexity and choice of the purification process depend on the degree of purity required: for example, removing small impurities by a simple procedure is appropriate for PCR use. However, all truncated sequences and shortmersmust be removed for use in qPCR or antisense applications.
As well as purity, the requirements for oligos imposed by the downstream application influence the acceptable yield and the nature of the oligo. This includes the length of the sequence and whether it is unmodified or modified. For qPCR or antisense applications, preparative HPLC is the most routinely employed technique, thanks to its high-resolution separation.
App Note: Streamlined Purification of Assay-Ready Oligonucleotides by Automated HPLC
Custom manufactured oligonucleotides require additional purification steps after synthesis to separate the full-length products from contaminants. The GX-271 Oligo Purification System can be used to isolate, analyze, and desalt reagent-grade oligonucleotides in one continuous run. Learn how in this application note.
Download App Note
Achieving High Purity with Preparative HPLC
Preparative HPLC encompasses several types of liquid chromatography that can be used to achieve a high level of oligo purity. To purify short to medium-sized oligos, a range of techniques are commonly used, either individually or in conjunction with one another:
- Anion exchange (AEX) HPLC
- Ion-pairing reversed phase HPLC (IP-RP HPLC)
- Gel filtration (size exclusion chromatography, SEC)
Here we explore each technique in more detail.
Anion Exchange Chromatography (AEX) HPLC
In AEX HPLC, a salt concentration gradient is used to progressively decrease interactions between the oligo and the column's cationic phase. The longer, more highly charged oligos are eluted and separated from shorter chain impurities. AEX HPLC is widely used to obtain unmodified oligos at high purity. Usually, these unmodified oligos will be up to 50-80 bases in length, depending on the desired purity.
Oligos contain a phosphate backbone, which affects how the purification is performed: the length of the oligo determines the quantity of negatively charged groups it contains.
Ion-Pairing Reverse Phase (IP-RP) HPLC
IP-RP HPLC is another chromatography technique to purify oligos, using an ion-pairing reagent such as triethylammonium acetate (TEAA). The approach utilizes differences in intrinsic hydrophobicity between full-length nucleotides —typically up to 40 bases —and the failure sequences for their effective separation. The mobile phase is a water/organic solvent mixture, where progressively increasing the elution strength elutes the full-length, modified oligo after any impurities. To improve the overall purity, the hydrophobic 5’-DMT group of the oligo can be left on, known as Trityl-On purification, and removed only after this purification step.
Compared to AEX HPLC, IP-RP HPLC is particularly useful for separating modified and unmodified oligos. It also offers improved separation resolution that is not achievable by cartridge-based purification. In addition, purified fractions produced by IP-RP HPLC are directly compatible with mass spectrometers, facilitating easier subsequent analysis.
Size Exclusion Chromatography (SEC)
Desalting is essential in purifying synthetic oligos, regardless of application, to remove salts and small-molecule impurities. Notably, remaining salts can interfere with subsequent analyses such as mass spectrometry, hindering accurate characterization. PCR applications also require precise buffer conditions that residual salts can disrupt.
There is a significant size difference between salts or other low molecular weight impurities and the oligonucleotides, which SEC can efficiently utilize for chromatographic desalting. Larger molecules can pass directly into the void volume of the resin, thereby eluting first, while smaller impurities must pass through the pore of the resin beads, delaying their elution.
SEC is particularly useful after HPLC IP-RP purification to remove TEEA, which is highly toxic to living cells and difficult to remove by other techniques such as precipitation. What’s more, SEC can be easily automated to desalt oligos before lyophilization.
Improve Preparative HPLC with Automation
The sheer quantity of potential by-products makes extensive oligo purification vital. However, not only can purification require multiple steps, but it often demands different columns and procedures, becoming a time-and cost-intensive process very quickly.
A flexible automated purification system capable of isolating, analyzing, and desalting reagent-grade oligos in one continuous run can streamline the purification process while reducing workspace and maintenance costs compared to a multi-HPLC system approach. Upgrading to an automated purification process reduces the number of manual steps required, which increases overall throughput and limits the risk of human error.
However, it is critical to select the right system configuration. Several criteria must be considered, including:
- The autonomy and the related level of automation needed
- The cost and the return on investment (ROI)
- The reliability, reproducibility, versatility, and scalability offered by the system
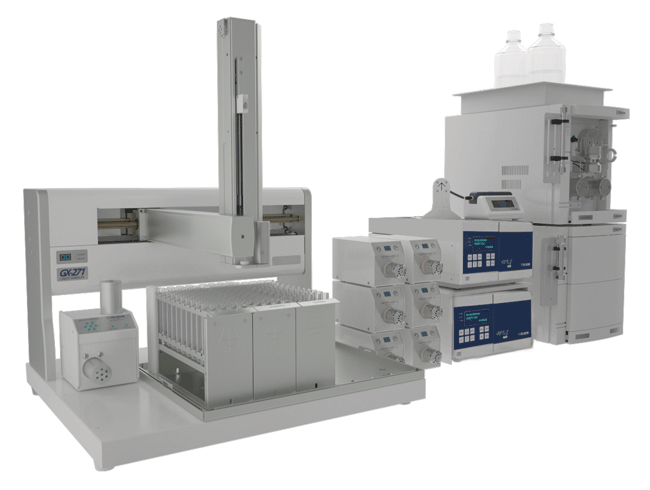
Figure 1. The VERITY Oligo Purification System is an all-in-one approach for continuous purification and analysis of reagent-grade nucleotides. The platform can be configured to meet your lab’s needs and budgets, offering capabilities from secure and robust purification to the continuous production of oligonucleotides. The modular system is also scalable to meet any future requirements.
Set Up for Future Success
When choosing an automated system for oligo purification, it is essential to consider your future needs. To maximize the applicability of the system to later workflows, you should carefully consider the five following points:
- What Needs Automating
You don’t need to automate everything, but it’s important to determine where automation will help your future workflow the most. Start by analyzing your current manual process and identify the steps that would benefit from automation. To select the most appropriate liquid handing/injection/fraction collection platform, determine the number of samples or steps that may need to be automated. Depending on the degree of automation you require, you can install one or more injection modules with automated HPLC column and mobile phase selection.
- Column Configuration
Ensuring flexible column configuration is the key to future success. In the most advanced automated HPLC installations, the required column (IEX, RP, or SEC) and the associated mobile phases can be automatically selected so multiple purification options can be done on the same platform. It is also possible to do analytical HPLC controls of the fractions collected online. You should consider a system that allows for automatic column regeneration, which will increase throughput and maximize the ROI for your new system.
- Reproducibility of Gradient Profiles
With automation, gradient profile reproducibility is crucial, but as your workflows change, the reproducibility needs to continue being consistent across a range of scales. Look for a system with a pump that can perform accurate and reproducible gradients from the analytical to the preparative scale.
- Multiple Wavelength Tracking
As lab priorities and workflows shift over time, you will likely need to track different wavelengths. Consider the UV-VIS detector of any system carefully and look for one featuring a multiple wavelength detector. These detectors trigger fraction collection, and their ability to track multiple wavelengths (e.g., 260, 290, 330 nm) combined with conditional collection operators (AND/OR/NOT) improve the reliability and quality of automated collection over a wider range of oligo concentrations, which may be critical for future work.
- Additional Modules and Software
Change in your workflows over time is inevitable, and for the most effective futureproofing, you should consider a system that enables different configurations and accommodates additional modules. In some systems, for example, pH–conductivity detectors can be added to control the quality of the gradient for AEX chromatography or salt removal during desalting.
Finally, while appropriate hardware is vital for your future workflows, the software is important, too. When choosing a new system, ensure the software enables safe and reproducible separations and trackability of your experiments.
One System, Mulitple Opportunities
The large number and variety of manual steps required for oligo purification —including preparation, analysis, and desalting —can be time-consuming, complicated, and costly. Choosing a versatile automated platform can significantly simplify oligo purification by bringing together all the different stages of oligo purification into a single system. The VERITY® Oligonucleotide Purification System allows you to handle all purification steps continuously, regardless of scale, to maximize product yield and efficiency and enable confidence in the integrity of your results.
Interested in Other Nucleic Acid Purification Applications?
Nucleic acid purification can also be used to describe the clean-up of nucleic acid samples, for example, to remove nucleotides and other contaminants from post PCR reactions. For the purpose of PCR, all primer sequences are chemically synthesized. These synthetic oligonucleotides can be purified by HPLC. Gilson has a range of applications and collaborations to assist and enable reproducible purification in this essential molecular biology procedure.
Explore Now